What is GMP Certification? A Guide for Supplement Manufacturers
The global dietary supplement market is expanding rapidly. This can be attributed to consumers in the United States and the United Kingdom who have become more health-conscious than ever before. They want to know exactly what is in their vitamins and protein powders. This is where GMP certification becomes a vital asset for any brand. It serves as a seal of quality and safety.
Defining GMP and its role in quality assurance
When someone asks what is a GMP certificate, they usually mean “How do I know this factory follows a quality system?” GMP stands for Good Manufacturing Practices (GMP guidelines). It covers how items are made, tested, labeled, and stored.
In the United Kingdom, the Food Standards Agency (FSA) and in the United States, the Food and Drug Administration (FDA) monitor these standards. The goal is to protect the consumer from products that do not work or could cause harm.
So, what is a GMP certificate? It is documentation issued after an audit that confirms that a facility meets the defined GMP certification requirements or the program scope. These standards cover every part of the production process. This includes raw materials, equipment used, and staff training.
GMP vs cGMP – What’s the Difference and Why It Matters?
The most common follow-up is: What is GMP certification compared with cGMP? cGMP means “current” Good Manufacturing Practices. “Current” points to modern controls and data integrity, especially in batch documentation.
This matters for online sales as static GMP guidelines might be outdated, and technology moves fast. The GMP certification requires companies to use modern laboratory equipment and software. If a company uses old methods, it might miss impurities that new machines would catch.
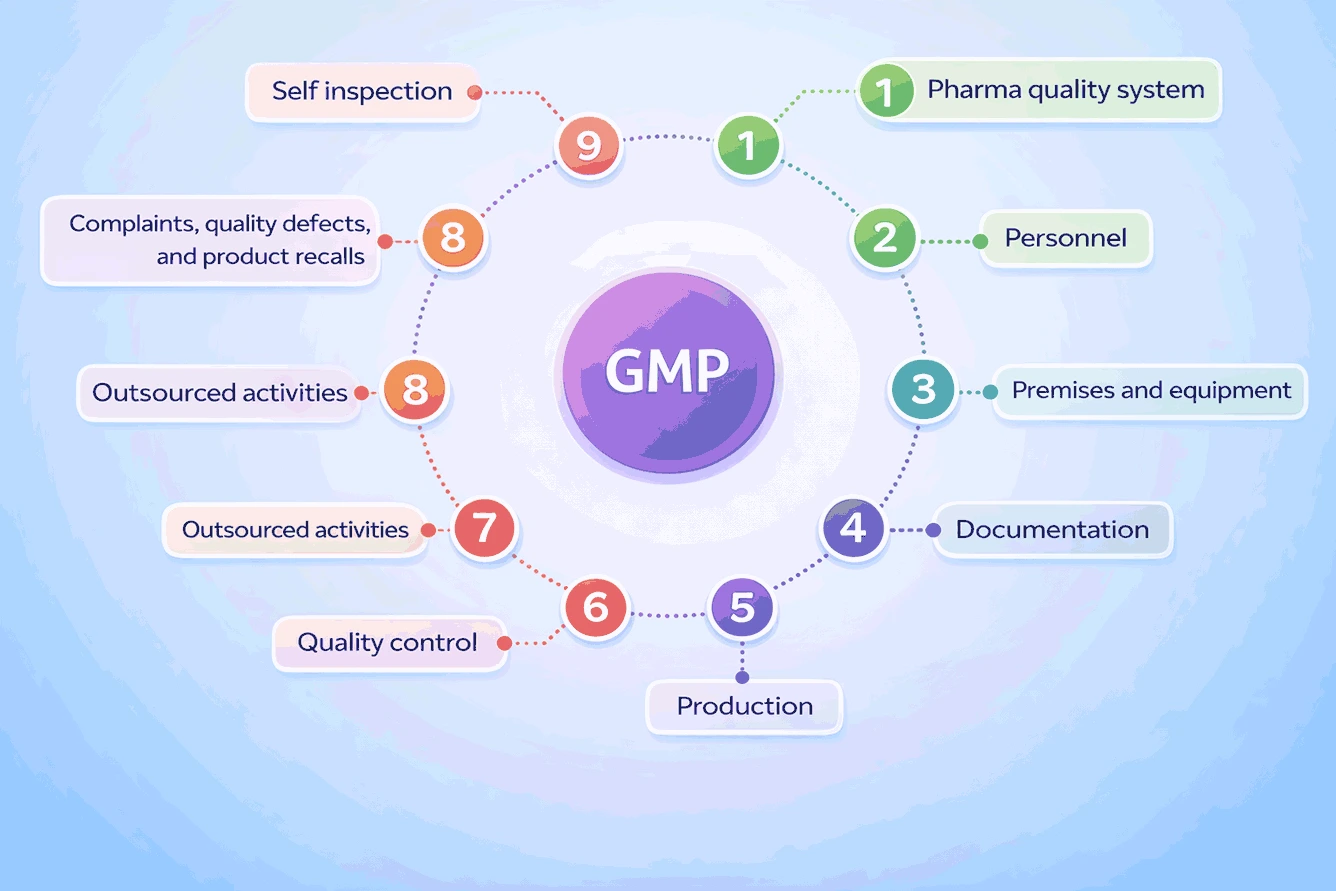
Key Components of GMP Compliance
Achieving GMP certification involves several steps. Here is a quick model of what is GMP certification, focusing on the four areas: the facility, the people, the process, and the records.
Manufacturing environment
The facility must support clean, controlled production and be designed to prevent cross-contamination. This includes air-handling systems, pest-control programs, and controlled material flow.
Auditors assess layout, equipment maintenance, sanitation records, and controlled material flow. You must note that poor facility design remains one of the most common audit findings.
Employee training and hygiene
People are an important part of the process, as they affect product quality as much as equipment does. Employees must receive regular training on GMP guidelines. They must wear protective gear to prevent human bacteria or hair from entering the product.
Moreover, the staff must understand hygiene rules, material handling, and deviation reporting. Clear protocols for handwashing and illness reporting are mandatory. Ongoing training is expected, not a one-time event.
Manufacturing process controls
Process controls are the step-by-step instructions that keep batches consistent. This includes material verification, weighing steps, blending times, and packaging checks.
Process controls define how each supplement is made. Clear procedures support repeatable results across batches. They also help teams respond faster during investigations or recalls.
The FDA rules require a master manufacturing record and a batch production record for each batch. These controls include ingredient checks, in-process inspections, and line clearance. For GMP certification supplements, process validation receives extra attention.
Batch records documentation
If a problem occurs, a company must be able to trace it back. Batch records act like a diary for every product made. They list the exact production time, the specific machines used, and the lot numbers of the raw ingredients. This makes it possible to perform a precise recall if a specific ingredient is found to be faulty.
Missing data, corrections without explanation, or late entries often lead to audit findings. Many facilities now use electronic systems to improve accuracy and review speed. Accurate records support traceability and regulatory confidence.
Why GMP certification is critical for dietary supplements
Brands must ask what is GMP certification because supplements sit under higher scrutiny than many consumer products. FDA compliance programs describe how inspections cover production steps and finished labels across multiple products.
In Great Britain, many supplements are treated as foods, so food hygiene controls apply. NHS guidance notes that food supplement makers follow food manufacturing standards, while medicines follow Medicines and Healthcare products Regulatory Agency (MHRA) standards. Food safety management procedures are commonly based on Hazard Analysis and Critical Control Points (HACCP) principles.
Recent market data shows that the majority of supplement users check labels for third-party certifications. A GMP certificate serves as a badge that conveys to the customer that the product contains what the label claims.
Common GMP audit findings and how to avoid them
Audits can be stressful, but if you want to understand what GMP certification supplements through an auditor’s lens, focus on common issues found during audits, such as:
Choosing a GMP certified manufacturer: What to look for
If you are a brand owner, you probably do not own a factory and are likely to partner with a contract manufacturer. You must verify that they follow GMP guidelines by asking for their current documentation and checking the dates.
Look for partners with high ratings from groups such as the National Sanitation Foundation (International) or Underwriters Laboratories (UL Solutions). These organizations are respected in both the US and the UK.
The GMP certification cost is often reflected in production costs. If a manufacturer is offering lower cost, they might be cutting corners. A higher price usually pays for the peace of mind that your products are safe and legal.
Why Choose F.A.M.E Health Labs for GMP-Compliant Manufacturing?
If your team keeps asking what is GMP certification and how to operationalize it, choose a partner that can quickly share documentation and explain it clearly. F.A.M.E Health Labs understands the challenges of the market and supports brands seeking GMP-aligned manufacturing, clear batch documentation, and audit-ready habits. We help you turn the question of what is a GMP certificate is into a powerful selling point for your customers.
Building a Brand Consumers Can Believe in with GMP certification
The health and wellness world is changing, with science and safety as top priorities for consumers. Understanding what is GMP certification is the first step toward building a successful supplement brand. It provides a clear framework for quality that everyone can trust. By following these rules, you protect your customers and your business. The future of supplements belongs to those who commit to the highest standards today.
FAQs
What does GMP certification mean?
It means that a manufacturing facility has passed an inspection. The inspection confirms they follow strict quality and safety standards.
Is GMP certification mandatory for supplements?
Yes. In the United States, it is a legal requirement under 21 CFR Part 111 of the FDA. In the United Kingdom, food safety laws require similar standards. While a third-party certificate is optional, following GMP guidelines is mandatory.
How long does it take and what does it cost to get GMP certified?
A first-time audit takes six months to a year. You should have a budget between $15,000 and $25,000 for a small plant and more than $50,000 for a large facility. It can cost up to $8,000 or more to run annual audits, verify continued compliance with GMP certification, supplement standards, and conduct facility upgrades and staff training.
How can I verify if a supplement manufacturer is GMP certified?
You can ask to see their GMP certificate. You can also check the online databases of certifying bodies. Organizations such as NSF, SQF, BRCGS online directory, and the ISA maintain public lists of certified facilities.



.png)



















.gif)


